Our validation service comply with regulatory requirements and quality standards. With 20 years of rich experience and expertise in biopharma, we offer a wide range of tests for your filters, single-use bags and assemblies.
Our Validation Center is established with an area of 7000㎡, approved and validated by CNAS . Currently we have more than 8 foreign laboratory experts and provide 6200 validation reports to biopharmaceutical customers every year.

-
10+
Laboratories
-
7000+
Area
-
54+
Experimental Methods
-
350+
Scientists and Technicians
-
30000+
Validation Reports Annually
-
Sterile Filtration Validation
Bacterial challenge test is a critical step for drug manufacturing and processing to validate final sterilizing-grade filters. Cobetter have 20 years of experience and technical expertise in sterile filtration validation.
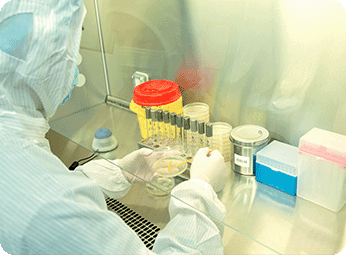
- Bacterial challenge test
- Chemical compatibility test
- Extractables and Leachables test
- Binding study
- Integrity test
-
Extractables and Leachables StudyWith the wide use of single-use systems in the biopharmaceutical industry, extractable and leachables (E&L) testing become very important.
TYPE Testing Methods Target Organic HS-GC-MS Volatile Substances DI-GC-MS Volatile and Semi-Volatile Substances UPLC-UV-MS Semi-Volatile, Non-Volatile Substances HPLC Semi-Volatile, Non-Volatile Substances TOC Total Organic Carbon FTIR Non-Volatile Residue Inorganic ICP-MS Inorganic Elements Organic/Inorganic NVR Non-Volatile Substances UV UV-Absorbing Substances pH Acidic or Alkaline Substances Conductivity Conductive Lons -
Virus Clearance ServicesVirus clearance study is the most important step of a biopharmaceutical manufacturer’s strategy to ensure drug product safety.?Virus clearance is the only way to prevent unexpected virus contamination, therefore, reliable virus clearance validation is crucial to ensuring patient safety.?Cobetter provide sufficient virus clearance data, as well as expert consultation on virus clearance process design and optimization.

- Official origin of cells and virus ensure traceablity of data
- From process development to VC validation
- Customizable services with high purity virus
- High purity virus
- Compliant with GLP management
- Compliant with FDA/EMA/NMPA audit standards
Contact Us
We have experienced validation project managers, helping you choose the right validation services, develop and implement appropriate validation strategies.
 eShop
eShop
 Sign in
Sign in English
English